Gastric cancer is the 15th most common type of cancer in the United States with over 26,000 new cases per year.1
NeoGenomics offers a comprehensive gastric cancer solution with tests detecting genomic alterations for diagnosis, therapy selection, prognosis, and clinical trial options. With many patients presenting with advanced disease, biomarker testing plays a crucial role in diagnosis, classification, and molecular characterization, enabling oncologists to understand tumor profiles and develop tailored treatment plans for gastric and gastroesophageal junction cancers. Testing options include next-generation sequencing (NGS), immunohistochemistry (IHC), and HER2 amplification assessment to support precision oncology. NeoGenomics provides essential therapy selection information through comprehensive genomic profiling (CGP).
Featured gastric cancer solutions
NEO PanTracer Tissue (formerly Neo Comprehensive - Solid Tumor)
Formerly Neo Comprehensive - Solid Tumor. NGS comprehensive genomic profile targeting 517 genes in DNA and RNA for SNVs, InDels, CNVs, RNA fusions/splice variants with MSI and TMB for pan-solid tumors.
8-10 Days turnaround time
NEO PanTracer LBx
Liquid biopsy NGS comprehensive genomic profile targeting 514 genes for SNVs, InDels, CNVs, and fusions with MSI-High and bTMB for advanced stage pan-solid tumors.
7 Days turnaround time
Comprehensive testing for advanced-stage gastric cancer patients
Testing patients with metastatic gastric cancer for actionable and emerging biomarkers can provide insight into a patient’s eligibility for targeted therapies.
PanTracer Tissue covers key biomarkers recommended by clinical practice guidelines.
Guideline-driven biomarker detection for gastric cancer2-5
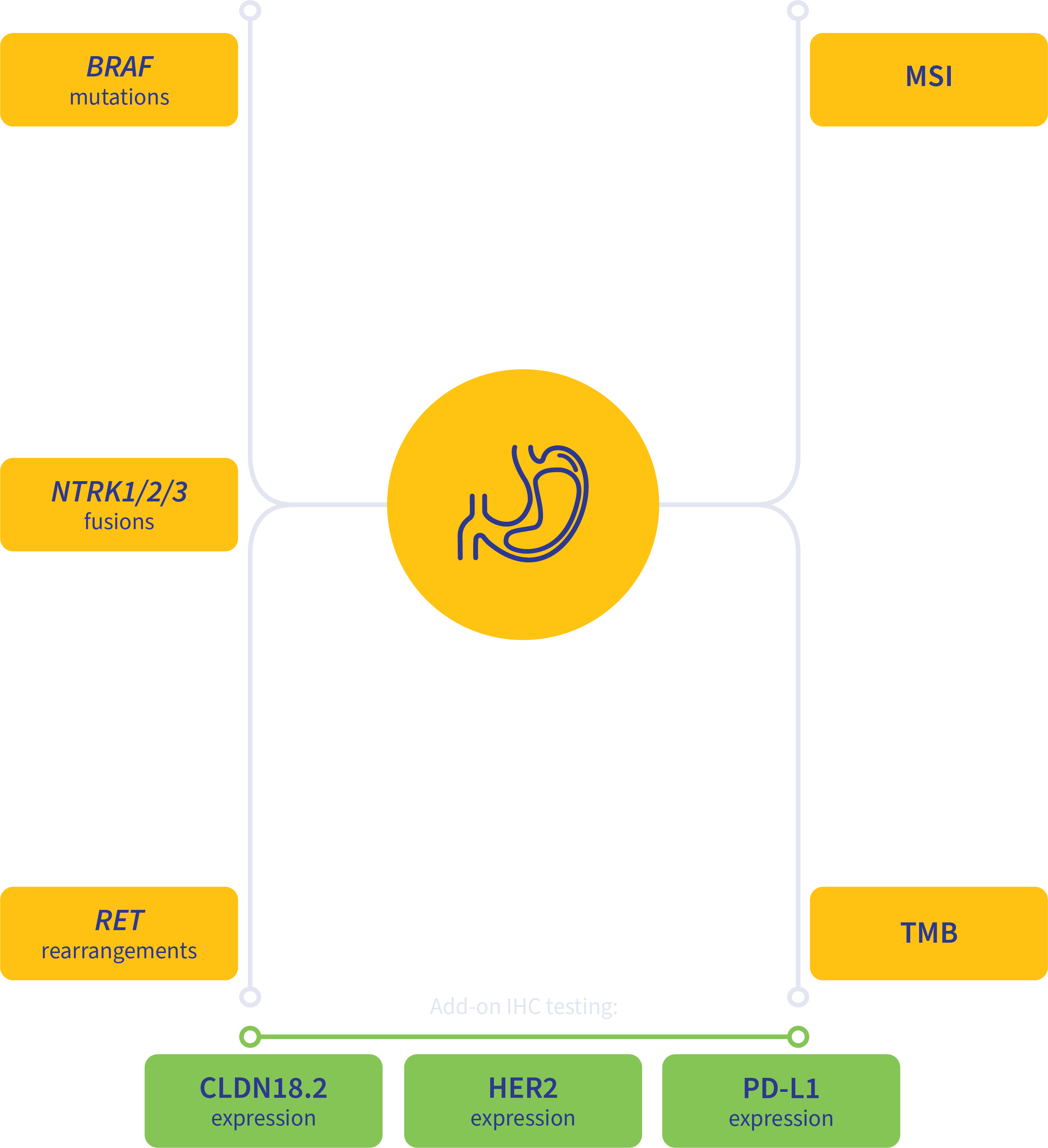
MSI, microsatellite instability; TMB, tumor mutation burden.
Add-on testing for therapy selection
Claudin 18 FDA (VYLOY®) for Gastric/GEJ Adenocarcinoma
1-2 Days turnaround time

Recently approved biomarkers– Claudin18.2
Claudin18 is a new actionable target for patients with advanced or metastatic gastric/GEJ adenocarcinoma who may be eligible for biomarker-driven therapy. NeoGenomics is one of the leading laboratories that offers Claudin18 testing immunohistochemistry (IHC).
Biomarker-informed decision-making reveals opportunities to advance care and provide insight on previously unknown patient populations. Claudin18.2 is a new companion biomarker, making it a useful tool for targeted therapy in combination with other standard genomic profiling.
Personalized patient care starts with precision oncology.
70% of gastric/GEJ cancers have Claudin18 at any expression level. 38% of patients have moderate to high expression levels of Claudin18.2 and may benefit from using a targeted biomarker assay to inform treatment decisions that may improve outcomes.6
References
National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. Cancer stat facts: stomach cancer. Accessed December 23, 2024. https://seer.cancer.gov/statfacts/html/stomach.html
Xia JY, Aadam AA. Advances in screening and detection of gastric cancer. J Surg Oncol. 2022;125(7):1104-1109.
Suehnholz SP, Nissan MH, Zhang H, et al. Quantifying the expanding landscape of clinical actionability for patients with cancer. Cancer Discov. 2024;14(1):49-65.
Chakravarty D, Gao J, Phillips S, et al. OncoKB: A precision oncology knowledge base. JCO Precis Oncol. 2017;2017:PO.17.00011.
Shah MA, Kennedy EB, Alarcon-Rozas AE, et al. Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO Guideline. J Clin Oncol. 2023;41(7):1470-1491.
Astellas Pharma Inc. Gastric cancer biomarkers: CLDN18.2. Accessed December 23, 2024.
https://www.gastriccancerbiomarkers.com/#cldn18-2-biomarker