In the United States, 1 in 8 women will be diagnosed with invasive breast cancer.1
Empowering providers with actionable insights, our comprehensive suite of tests to support the care continuum from diagnosis to therapy selection, prognosis, and clinical trial options.
Featured breast cancer solutions
NEO PanTracer Tissue (formerly Neo Comprehensive - Solid Tumor)
Formerly Neo Comprehensive - Solid Tumor. NGS comprehensive genomic profile targeting 517 genes in DNA and RNA for SNVs, InDels, CNVs, RNA fusions/splice variants with MSI and TMB for pan-solid tumors.
8-10 Days turnaround time
Breast Cancer Index® (BCI)
NeoGenomics has partnered with Biotheranostics, a Hologic Company, to make ordering BCI easy for women with early-stage hormone receptor-positive (HR+) breast cancer. BCI is the only test recognized by the NCCN® Guidelines to predict which patients are likely to benefit from extended adjuvant anti-estrogen therapy beyond 5 years.
10 Days turnaround time
NEO PanTracer LBx
Liquid biopsy NGS comprehensive genomic profile targeting 514 genes for SNVs, InDels, CNVs, and fusions with MSI-High and bTMB for advanced stage pan-solid tumors.
7 Days turnaround time
Comprehensive testing for metastatic breast cancer patients
Clinical guidelines recommend biomarker testing for all metastatic breast caner patients, regardless of subtype.2
Studies have shown that potentially actionable genomic alterations can be identified in over 70% of metastatic breast cancer patients when tested with a comprehensive, next generation sequencing panel.3
PanTracer Tissue covers key biomarkers recommended by clinical practice guidelines.
Guideline-driven biomarker detection for advanced-stage breast cancer2,4-8
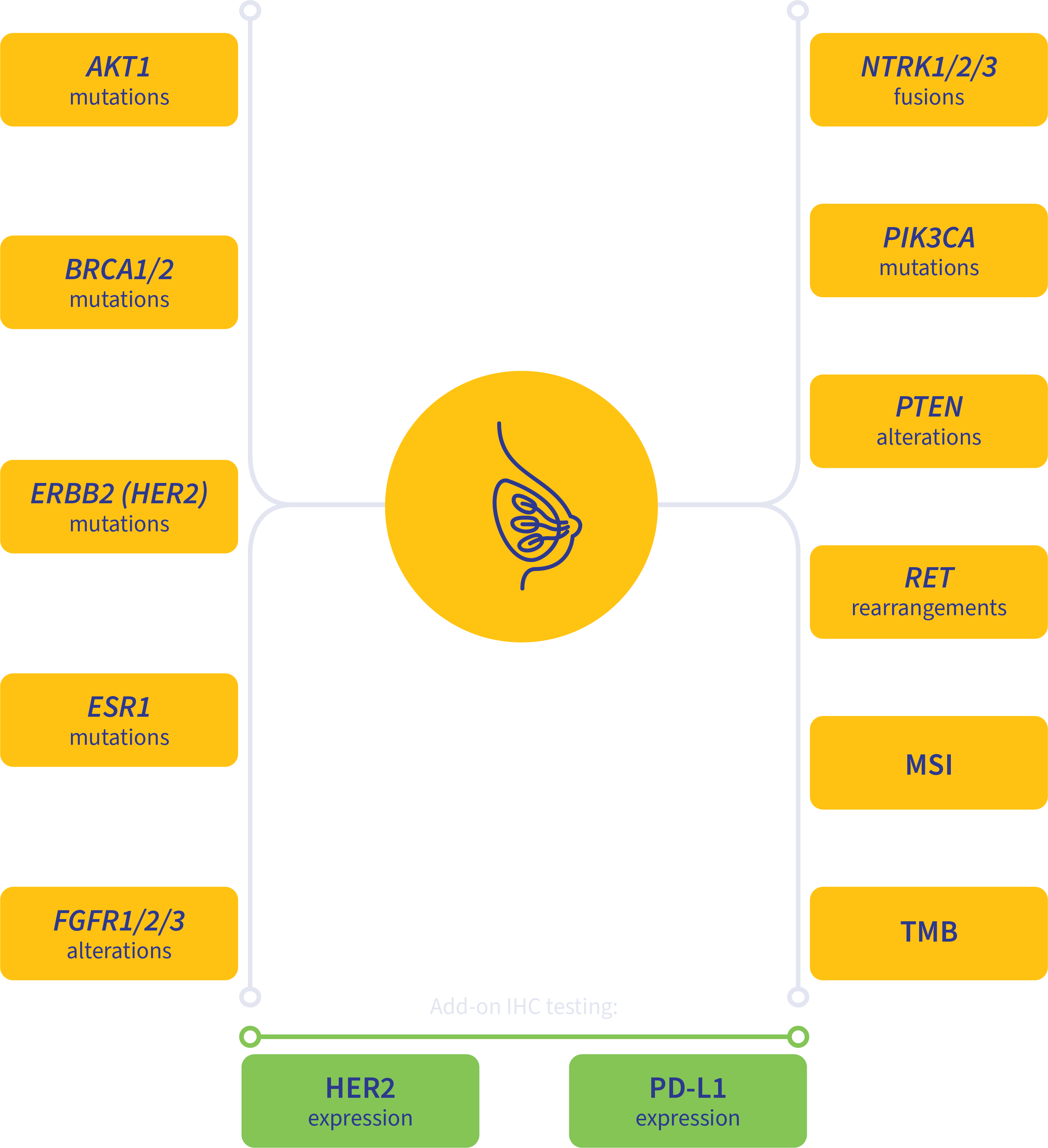
MSI, microsatellite instability; TMB, tumor mutation burden.
Add-on testing for therapy selection
References
Menon G, Alkabban FM, Ferguson T. Breast cancer. StatPearls [Internet]. StatPearls Publishing. Last updated February 25, 2024. Accessed December 23, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482286/
Dietrich M, Velez M. Larotrectinib in NTRK3 fusion–positive metastatic secretory carcinoma of the breast: A case study. Curr Probl Cancer Case Rep. 2025;17:100334.doi:10.1016/j.cpccr.2024.100334
van Geelen CT, Savas P, Teo ZL, et al. Clinical implications of prospective genomic profiling of metastatic breast cancer patients [published correction appears in Breast CancerRes. 2022 Jul 15;24(1):50. doi: 10.1186/s13058-022-01539-7.]. Breast Cancer Res. 2020;22(1):91. Published 2020 Aug 18. doi:10.1186/s13058-020-01328-0
Bhave MA et al. Comprehensive genomic profiling of ESR1, PIK3CA, AKT1, and PTEN in HR(+)HER2(-) metastatic breast cancer: prevalence along treatment course and predictivevalue for endocrine therapy resistance in real-world practice. Breast Cancer Res Treat. 2024;207(3):599-609. doi:10.1007/s10549-024-07376-w
Varga Z, et al. Molecular pathology in breast disease: diagnostic, prognostic, and therapeutic tools. Virchows Arch. 2024;484(2):247-261. doi:10.1007/s00428-023-03709
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) 2023 Updates. The ASCO Post. Published May 25, 2023.
Genomenon. FGFR2 Gene. CKB. Accessed March 26, 2025. https://ckb.genomenon.com/gene/show?geneId=2263
Badve SS, et al. Determining PD-L1 Status in Patients With Triple-Negative Breast Cancer: Lessons Learned From IMpassion130. J Natl Cancer Inst. 2022;114(5):664-675.doi:10.1093/jnci/djab121