Lung cancer impacts 1 in 17 men and 1 in 18 women.1
NeoGenomics offers a comprehensive lung cancer solution that supports the care continuum with tests that detect genomic alterations for diagnosis, therapy selection, prognosis, and clinical trial options. We meet you and your patients where they are. Whether their disease is newly diagnosed or recurrent, our lung cancer solution aims to provide the information you need to treat with confidence. While the treatment landscape for advanced-stage non-small cell lung cancer (aNSCLC) has grown increasingly complex, NeoGenomics aims to simplify biomarker testing by offering both tissue and liquid biopsy comprehensive genomic profiling (CGP) options that take the guesswork out of the process.
Featured lung cancer solutions
NEO PanTracer Tissue (formerly Neo Comprehensive - Solid Tumor)
Formerly Neo Comprehensive - Solid Tumor. NGS comprehensive genomic profile targeting 517 genes in DNA and RNA for SNVs, InDels, CNVs, RNA fusions/splice variants with MSI and TMB for pan-solid tumors.
8-10 Days turnaround time
NEO PanTracer LBx
Liquid biopsy NGS comprehensive genomic profile targeting 514 genes for SNVs, InDels, CNVs, and fusions with MSI-High and bTMB for advanced stage pan-solid tumors.
7 Days turnaround time
Early-stage NSCLC Panel
The Early-stage NSCLC Panel analyzes 4 relevant and actionable biomarkers through a combination of multi-modality methods: EGFR (PCR), ALK (FISH), ROS1 (FISH), PD-L1 22C3 (IHC).
7 Days turnaround time
Comprehensive testing for aNSCLC patients
In the era of precision medicine, the number of FDA-approved targeted therapy options has accelerated; this is particularly true for lung cancer. Up to 70% of advanced-stage NSCLC patients have a known oncogenic driver mutation, many of which are associated with an approved therapy.2 Comprehensive profiling with next-generation sequencing identifies targetable genomic alterations and ensures the best therapy is provided to the patient, improving patient outcomes.
The PanTracer portfolio provides CGP testing from either tissue or liquid biopsy samples. The broad panels cover key biomarkers recommended by clinical practice guidelines.
PanTracer Tissue and PanTracer LBx can be ordered individually or together to maximize genomic insights.

Concurrent testing
PanTracer Tissue and PanTracer LBx can be ordered either simultaneously or as an automated reflex when tissue testing fails.
Simultaneous tissue — and liquid-based testing is a practical and time-saving method for identifying actionable biomarkers for therapy decisions, ensuring fully informed choices for the best possible patient outcomes by expediting the time to treatment and reducing delays due to insufficient tissue.
Guideline-driven biomarker detection for lung cancer3-6
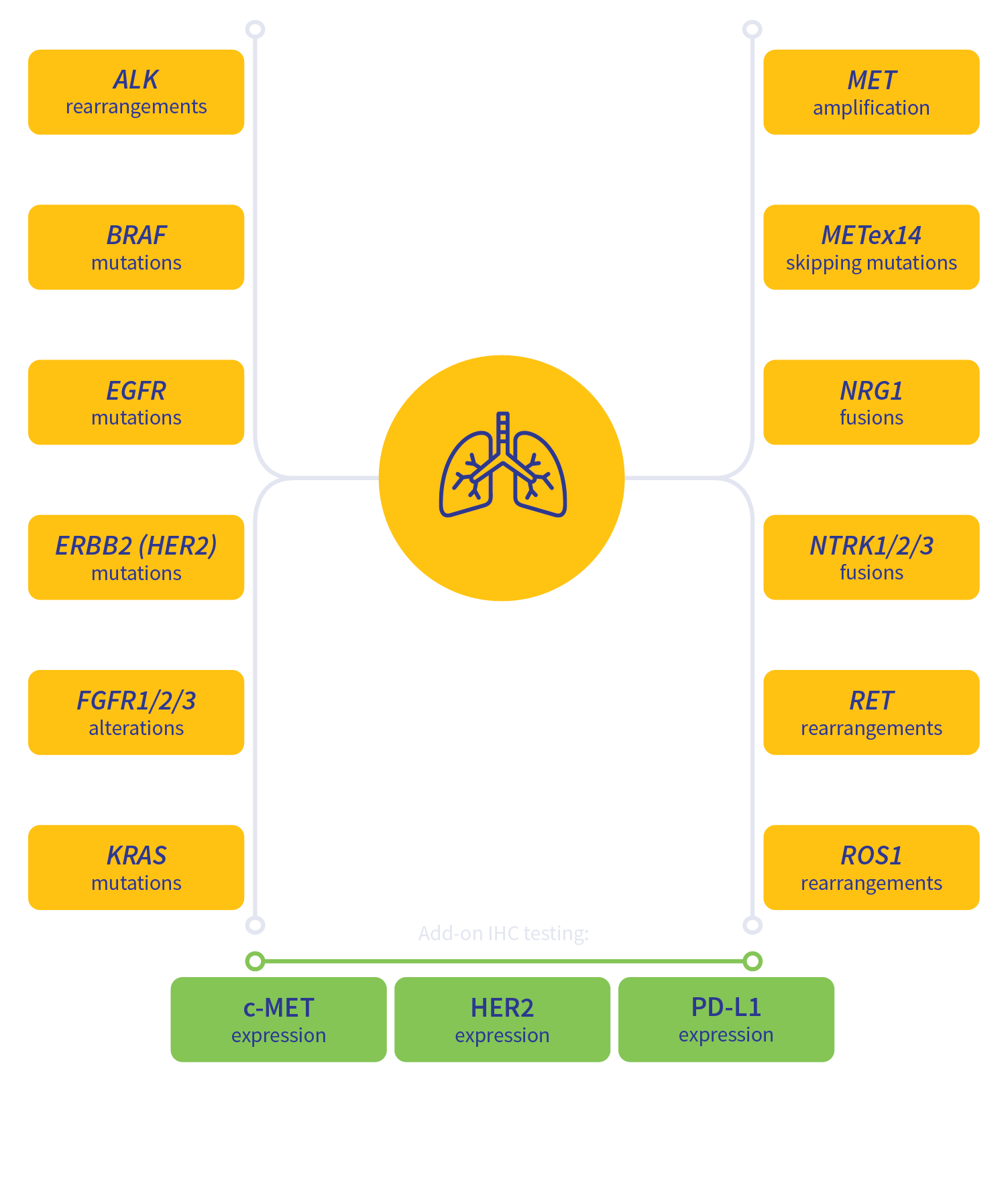
MSI, microsatellite instability; TMB, tumor mutation burden.
Add-on testing for therapy selection
c-MET CDx for NSCLC
Global: 2 Day, Tech-Only (stain only): 1 Day turnaround time

Recent advancements in targeted therapy for NSCLC patients:
c-MET is a new actionable biomarker with an FDA-approved targeted therapy for NSCLC patients.
In the era of precision medicine, the number of FDA-approved targeted therapy options has accelerated; this is particularly true for lung cancer.1 c-MET is a new actionable biomarker with an FDA-approved targeted therapy for NSCLC patients that could improve patient outcomes.
NeoGenomics is proud to offer c-MET CDx for NSCLC to support precision oncology in non-small cell lung cancer (NSCLC). This advanced immunohistochemistry (IHC) assay enables precise detection of MET protein expression, empowering oncologists to identify patients with NSCLC who may benefit from a new targeted therapy.
My liquid biopsy test has potentially bought me several more years that I wouldn’t have had otherwise. That’s something that money can’t buy.
References
American Cancer Society. Key Statistics for Lung Cancer. American Cancer Society. Updated January 16, 2025. Accessed August 14, 2025. https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html
Hirsch FR, Suda K, Wiens J, Bunn PA, Jr. New and emerging targeted treatments in advanced non-small-cell-lung-cancer. Lancet. Sep 3 2016.388(10048): 1012-1024.
Moore DC, et al. The role of biomarkers in guiding clinical decision-making in oncology. J Adv Pract Oncol. 2023;14(3):241-248.
SSpacegen. New markers of lung cancer seen from the 2025 NSCLC NCCN guideline V1. Published December 30, 2025. Accessed March 25,2025. https://sspacegen.com/en/newsinfo.aspx?newsID=417&CateId=109&parentid=0
Yang M, et al. Non-small cell lung cancer with MET amplification: review of epidemiology, associated disease characteristics, testingprocedures, burden, and treatments. Front Oncol. 2024;13:1241402.
U.S. Food and Drug Administration. FDA grants accelerated approval to telisotuzumab vedotin (TeliV) for NSCLC with high c-Met protein overexpression. Published July 2, 2025. Accessed July 10, 2025.
https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approvaltelisotuzumab-
vedotin-tllv-nsclc-high-c-met-protein-overexpression